Hindered amine light stabilizers (HALS) are important stabilizers for long-term thermal protection of polymers, and are very effective in inhibiting polymer degradation caused by free radicals at medium and low temperatures.
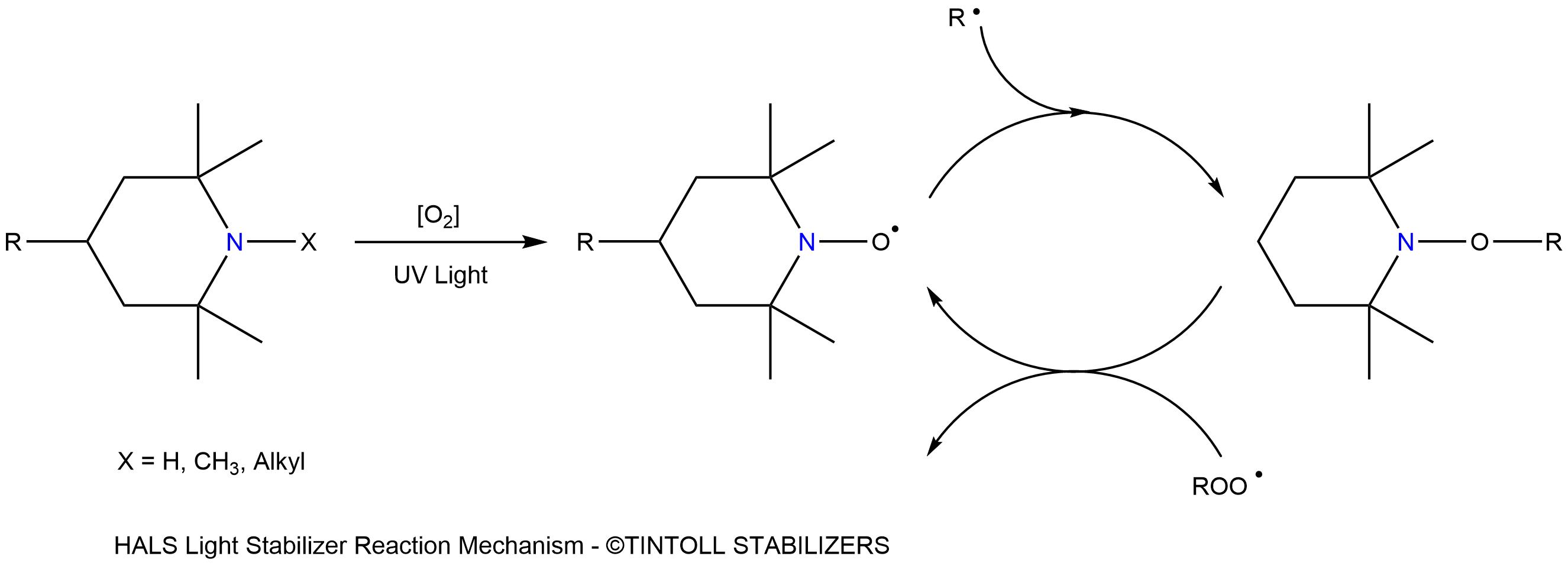
Hindered Amine Light Stabilizers (HALS) exhibit excellent UV protection by scavenging free radicals generated by photodegradation to prevent polymer degradation. After the chemical scavenges free radicals, it returns to its original structure and repeatedly scavenges free radicals for effective long-term performance.
HALS can be classified according to their molecular weight (MW): HALS with a low molecular weight of approximately 200 to 500 g/mole are generally referred to as low MW HALS, while HALS with a molecular weight of 2000 or higher are referred to as high MW HALS. In general, high molecular weight hindered amine stabilizers are more effective than low molecular weight hindered amine stabilizers, and very low molecular weight hindered amine stabilizers do not provide much thermal stability at all.
The high efficiency of HALS is based on a series of complex free radical scavenging reactions. HALS oxidation is a relatively slow and temperature-dependent reaction. They are not very effective at high temperatures (above about 80°C), HALS are often used in combination with primary and secondary antioxidants, so that the combination can show a good synergistic effect.
For more than 30 years, TINTOLL has been committed to providing innovative high-performance light stabilizer and antioxidant solutions to meet the growing and changing technical needs of the global plastics market.
HALS do not absorb UV radiation, but inhibit polymer degradation. They slow photochemically induced degradation reactions to some extent similar to antioxidants.
One of the advantages of HALS is that no specific layer thickness or concentration limits are required to guarantee good results. Remarkable plateaus are achieved at relatively low concentrations. The high efficiency and long life of HALS is due to a cyclic process where HALS are regenerated rather than consumed during stabilization.
The mechanism by which hindered amine stabilizers resist thermal oxidation appears to be complex. Due to the regenerative nature of the process, and the generally high molecular weight of the light stabilizers, hindered amine stabilizers can provide extremely long-term thermal and light stability.
The mechanism of HALS involves several different reactions that lead to their stabilization against photooxidation. The radical-scavenging properties of carbon radicals, the reaction of HALS metabolites with peroxyl radicals, and the decomposition of hydroperoxides have been discovered, as follows.